Chapter 16 Muscle Contraction and Related Phenomena
Unraveling Muscle Contraction: Ling's Paradigm Shift from Sliding Filaments to Osmotic Forces and ATP Dynamics
As always, this is not medical advice, and reading this does not form a client relationship with me - your health is your responsibility.
Many have asked for my “thoughts” on things. The best place to start would be some of my older Substacks - especially the ones on supplementation (1 and 2). Also, please check out my IG story highlights.
Today’s Substack will continue with Chapter 16, Muscle Contraction and Related Phenomena. Chapter 15 discussed oxidative phosphorylation, ATP synthesis, and other aspects of mitochondrial physiology.
Summary
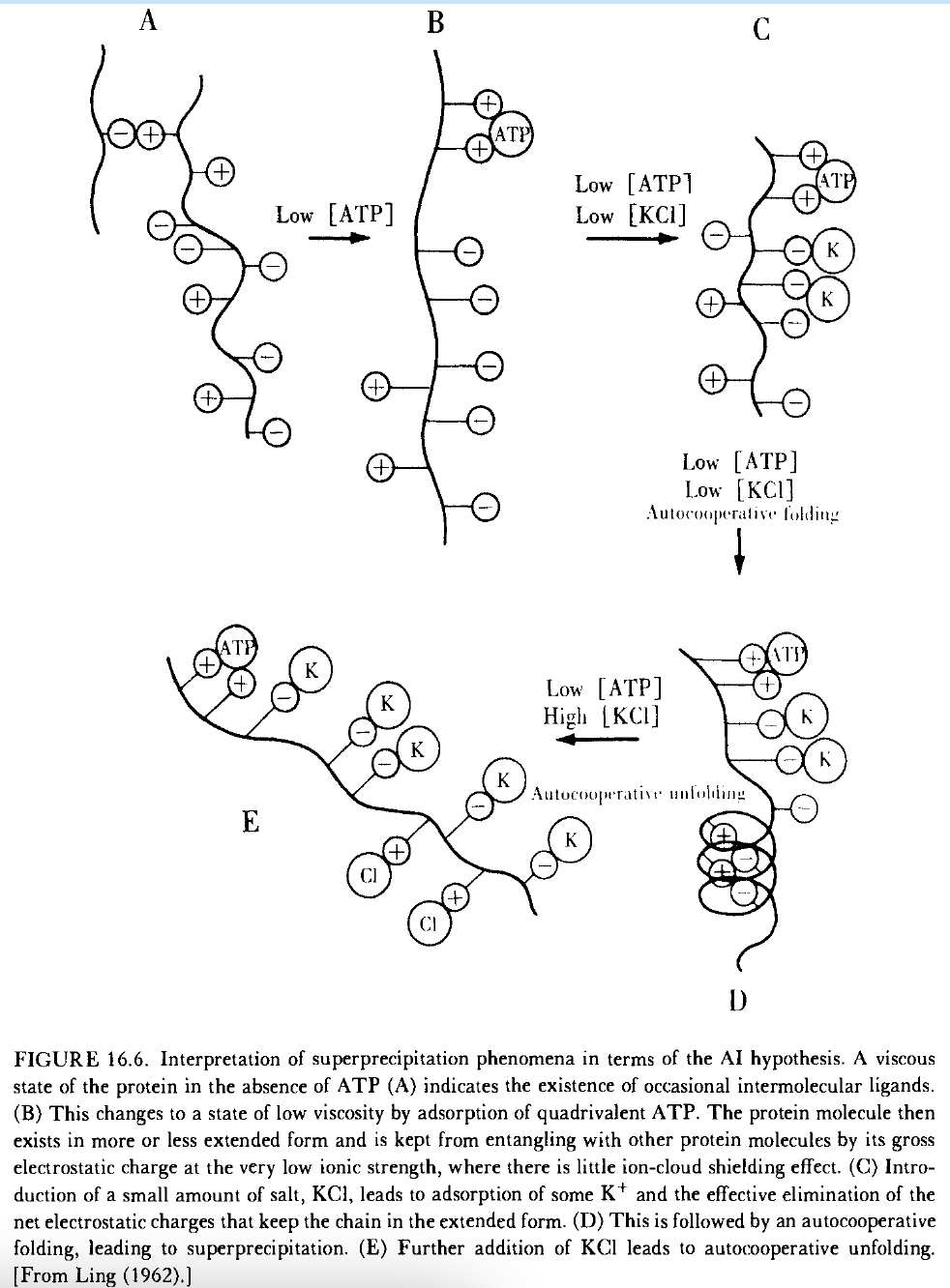
Muscles shorten when they contract and lengthen when they relax. The skeletal muscle contraction-relaxation cycle requires: actin, myosin, tropomyosin (binds to actin), troponin (binds Ca+2), Ca+2, ATP, binding or sequestration of Ca+2, K+, and a means of excitation. The sliding filament model is the accepted model governing the cycle. Tension is generated in the sliding filament model when myosin and actin filaments overlap and slide past each other, forming cross-bridges. Questions remain about the mechanism, including the interaction of actin and myosin during sliding, the expanded space between filaments during contraction, K+ release, regulation of ATPase activity, and the role of tropomyosin and troponin in Ca+2 sensitivity. An alternative approach, the association-induction hypothesis, considers muscle contraction from a different perspective. Key observations include the adsorption of K+ onto myosin carboxyl groups, polarized multilayers of muscle water interacting with actin, and ATP conditioning the autocooperative K+-adsorbing state and water-polarizing state. ATP's role in work performance involves adsorption onto key proteins, triggering a drop in high-potential energy of the relaxed muscle to a low-energy contracted state. Ca+2, another cardinal adsorbent, conditions the autocooperative K+-adsorption process. Muscle relaxation involves the dissociation of salt linkages, ATP hydrolysis to ADP, and the release of K+ and anions, leading to the formation of interprotein salt linkages and the rigid state of contracted muscle. Localized changes in water osmotic activity, along with K+ release and water depolarization, are postulated to provide the major force for muscle contraction. Despite advancements, many unresolved problems persist in understanding muscle contraction, relaxation, and related phenomena in nonmuscle cells. Take home: the potential energy comes from the entire cell and is converted to mechanical work.
“[T]he skeletal structure should counteract the pull of gravity, leaving the muscles free for movement. The nervous system and the frame develop together under the influence of gravity in such a way that the skeleton will hold up the body without expending energy despite the pull of gravity. If, on the other hand, the muscles have to carry out the job of the skeleton, not only do they use energy needlessly, but they are then prevented from carrying out their main function of changing the position of the body, that is, of movement.” - Moshe Feldenkrais
A light microscope shows the “alternatingly dark (anisotropic) A bands and light (isotropic) I bands” of a muscle fiber. Isotropic means its properties are the same in all directions. Thus, anisotropic means the properties are direction-dependent. Myosin is the major protein found in muscle cells. Actin is a major component of the cytoskeleton and along with myosin is responsible for muscle contraction. There is no change in cell volume during a muscle contraction.
16.1 Early Theories of Muscle Contraction
16.1.1. Engelmann's Heat Engine Theory: Engelmann proposed that the A band's doubly refractile material, when heated, undergoes a shape change that “is accompanied by an increase association with water” and lateral expansion, causing shortening of the I band. However, this “heat engine” theory was thermodynamically untenable. “The efficiency of muscle interaction is usually given as 30%” - imagine if you could get more, but the connective tissue needs to be taken into account.
16.1.2. The Osmotic Theories of McDougall and MacDonald: McDougall suggested that lactic acid increases osmotic activity in the A bands, causing lateral swelling and myofibril (= sarcostyles, the contractile element of muscle that includes actin and myosin) shortening. MacDonald proposed a different osmotic theory involving the movement of water between I and A bands.
16.1.3. The Lactic Acid Theory: Early beliefs centered around lactic acid or H+ causing a change in surface tension at the boundary layer between myofibrils and the sarcoplasm (= cytoplasm of a muscle cell) role in muscle contraction. This was not the case, as lactic acid production occurred after the contraction. Also, muscles that have been poisoned to not allow the production of lactic acid can still contract.
16.1.4. The Engelhardt-Ljubimova Theory: Weber and later Engelhardt and Ljubimova demonstrated that myosin lengthens in response to ATP hydrolyzation (= water breaks one of the phosphate bonds and ATP → ADP). However, other compounds also caused myosin relaxation - including thiamine pyrophosphate, which does not hydrolyze ATP.
16.1.5. The Actin-Myosin Association Theory of Szent-Gyorgyi: Albert Szent-Gyorgyi's studies revealed that myosin is not pure and can be isolated in two forms (A and B). Myosin A has more actin than myosin B. ATP causes the dissociation of actin and myosin. The actin-myosin association theory suggests that muscle contraction involves the association and dissociation of actin and myosin - actin and myosin are dissociated in a resting cell and associate to contract. ATP hydrolysis was not involved in this theory.
16.1.6. The Active Relaxation Theory: Muscles poisoned with iodoacetic acid (IAA) and nitrogen can contract normally until ATP and creatine phosphate are depleted, leading to rigor - “an extreme and irreversible form of contraction” (how many have muscle/ eye twitches, cramps, etc.?). This is an important point, a contraction is from a loss of potential energy, not a gain. It takes energy to keep the muscle in an uncontracted state. The contracted state is an excited state. The energy liberated from ATP hydrolysis is proposed to be utilized in the relaxation of the discharged state.
16.1.7. The Electrostatic Extension-Entropic Contraction Theory: The theory suggests that electric charges of ionized carboxyl groups in muscle proteins may repel each other, maintaining the muscle in a relaxed state. Neutralization of these charges would cause contraction. “ATP, adsorbed on the contractile proteins, may provide enough repellent force between them to maintain the muscle proteins in a relaxed, extended state. Hydrolysis of ATP and its desorption may then lead to contraction.”
16.1.8. The Earlier Association-Induction Model: This model, different from others, incorporates intracellular K+ in an adsorbed state and suggests that ATP acts as a cardinal adsorbent, keeping muscles in a lengthened, relaxed state via “direct electrostatic repulsion.” Contraction involves the formation of salt linkages between the negatively charged beta- and gamma-carboxyl groups displacing adsorbed K+. A relaxed muscle is elasti and a contracted more rigid - like liquid to solid. Cooling slows down ATP decomposition in a poisoned muscle - makes one wonder why so many seem to “need” ice baths nowadays.
16.2 Current View of the Mechanism of Muscle Contraction
Sliding Filament and Excitation-Contraction Coupling: The sliding filament theory is widely accepted as the mechanism of muscle contraction. According to this theory, muscle contraction occurs as a result of the sliding movement between the thin filaments (primarily composed of actin) and thick filaments (primarily composed of myosin) within the muscle fibers. This sliding action is powered by the interaction between myosin and actin filaments, facilitated by the hydrolysis of ATP (adenosine triphosphate) and the release of calcium ions.
During muscle contraction, calcium ions are released from the sarcoplasmic reticulum, and they bind to troponin, a regulatory protein associated with the actin filaments. This binding causes a conformational change in troponin and tropomyosin (another regulatory protein), exposing the active binding sites on actin. Myosin heads then bind to these exposed sites, forming cross-bridges.
ATP is hydrolyzed by the myosin heads, providing the energy required for the myosin heads to pivot and pull the actin filaments toward the center of the sarcomere (the basic structural unit of a muscle). This movement is often described as the sliding of the filaments past each other. After the power stroke, ATP binds to the myosin head, causing it to detach from actin. The cycle repeats as long as calcium ions are available, allowing muscle contraction to continue.
Resting State and Sarcomere Structure:
Muscles are in a relaxed state, with actin and myosin filaments partially overlapping in sarcomeres.
ATP molecules are bound to myosin heads, storing potential energy.
Excitation:
Nerve impulses, or action potentials, travel along motor neurons to reach neuromuscular junctions.
Acetylcholine is released, stimulating muscle cell membrane (sarcolemma) depolarization.
Action Potential Propagation:
The action potential spreads along the sarcolemma and into the transverse tubules (T-tubules).
Calcium Ion Release:
The depolarization triggers the opening of voltage-gated calcium channels in the sarcoplasmic reticulum (SR).
Calcium ions are released into the cytoplasm of the muscle cell.
Troponin and Tropomyosin Interaction:
Calcium binds to troponin, causing a conformational change.
Tropomyosin is shifted, exposing the myosin-binding sites on actin filaments.
Cross-Bridge Formation (Sliding Filament Theory):
Myosin heads bind to exposed sites on actin, forming cross-bridges.
ATP hydrolysis provides energy, causing myosin heads to pivot and slide actin filaments.
Power Stroke and Sarcomere Contraction:
The power stroke involves the physical movement of actin filaments toward the center of the sarcomere.
Sarcomeres shorten as actin and myosin filaments slide past each other.
ATP-Driven Cycling:
ATP binds to myosin heads, allowing them to detach from actin.
ATP hydrolysis (ATP → ADP + Pi) resets myosin heads, preparing them for the next cycle.
Calcium Ion Removal:
Calcium ions are actively transported back into the sarcoplasmic reticulum, reducing cytoplasmic calcium concentration.
Muscle Relaxation:
With decreased calcium concentration, troponin and tropomyosin return to their original positions, blocking myosin-binding sites on actin.
Other Theories: It is not clear how “ATP energizes work performance.” The sliding filament model was meant to only explain striated muscle contraction - cardiac and skeletal muscle, not smooth muscle. Smooth muscle is found in the walls of various internal organs such as the digestive tract, blood vessels, respiratory passages, and reproductive organs and is usually involuntary and under the control of the autonomic nervous system. Attempts to extend the sliding filament theory to other smooth muscle and other systems have been met with limited success.
“Muscle contraction follows a reduction of the attractive forces between the myofilaments, forces which in resting muscle hold in check a natural tendency toward lateral expansion owing to repulsive long-range forces.” Water was said to maintain the attractive force overcoming the repulsion. Once the water structure was degraded, lateral expansion would occur, and the muscle cells would shorten.
Electrostatic-hydraulic theory, where electrostatic repulsion forces between filaments cause shortening, sustained by frictional resistance to water flow from protein filament hydration.
“[T]hick and thin filaments carry opposite electric charges,” generating a force to maximize filament overlap.
Another electrostatic theory came about because in contracting muscle, thin filaments, having a low dielectric constant and high electric conductance, generate a force pulling them in between thick filaments. The dielectric constant, also known as relative permittivity, quantifies a material's ability to store electrical energy in an electric field. High electric conductance refers to the capacity of a material to allow the flow of electric current with minimal resistance.
16.3 Critique of the Sliding Filament Model
Given how complex and beautiful the human body is, no one model will adequately describe an action, system, or the whole.
16.3.1. The Energy Problem: The energy from the hydrolysis of ATP is insufficient for muscle contraction and other work done in living cells.
16.3.2. The Number, Duration, and Synchronization of Cycles of Cross-Bridge Formation and Breakage: At 0C each muscle twitch takes 200ms, at body temperature this occurs even faster, <37ms. Cross-bridge formation and synchronization takes too long. There must be perfect cross-bridge dissociation synchrony so one or more does not act as an anchor which would prevent filament sliding (= the muscle does not shorten). All the cross-bridges must form to allow for sufficient tension to lift the weight.
The calcium oscillations also pose an issue as the Ca+2 pump may be sufficiently fast to remove the liberated Ca+2 during the previous cycle. However, the return of the Ca+2 is rate-limited by uncontrollable diffusion.
16.3.3. What Keeps the Filaments from Tangling Up?: There is no known mechanism that would stop the thin filaments from becoming tangled.
16.3.4. Why Should the Bulk of Water in the I Bands Move with the Telescoping Thin Filaments?: Living cells are 80% water by weight. If that water is free from the actin thin filaments, one would expect the water to move during a contraction - the volume has changed and liquids fill volume. There is no barrier that would prevent the water from squeezing between the myofibrils. Instead, the water follows the thin filaments, as if it were bonded to them.
16.4 A Tentative Model of Muscle (and Nonmuscle Cell) Contraction: An Updated Theory According to the AI Hypothesis
16.4.1. The Resting, Relaxed Muscle: In the resting muscle, actin is suggested to exist in a "profilamentous" state, with a substantial part exposed to bulk phase water. This state polarizes water into multilayers, maintaining osmotic equilibrium. Contrary to conventional thinking, the low K+ and ionic strength inside resting cells suggest that actin does not exist in the F-actin form but rather in the "profilamentous" form.
Ling sees myosin in the conventional view - “thick filaments and the myosin heads undergo confirmation changes and form cross-bridges. ATP as a cardinal adsorbent keeps neighboring anionic sites… favoring K+ adsorption".”
“[T]he bulk of muscle K+ is adsorbed at the two edges of the A band, in aread where most of the myosin heads are located.”
“[The] potential energy involving all the associated components of the resting protoplasm… provides the energy for contraction. ATP’s contribution is that of a cardinal adsorbent.” Yes, this means the protoplasm energy and information transfer is the most important function of the cell.
16.4.2. Contraction:
The action potential triggers Ca+2 release. The Ca+2 combines with troponin. Troponin is a regulatory protein complex that is part of the troponin-tropomyosin complex. Ca+2 acts as a cardinal adsorbent which leads to the combination of prepolymerized F-actin with nearby myosin heads, activating myosin ATPase. The myosin ATPase hydrolyzes ATP, the other cardinal adsorbent.
ATP hydrolysis releases ADP and inorganic phosphate. This liberates K+ and forms cross-bridges via salt linkage between actin and myosin heads.
The local effects of K+ liberation and actin polymerization result in water flow, causing lateral expansion and longitudinal shortening.
Filamentous actin “combines with more myosin heads distally, activating its ATPase, and the cycle” continues, leading to the growth of thin filaments and the typical "sliding filaments" pattern in contraction.
16.4.3. Relaxation:
With no additional nerve impulse, the protoplasm returns to its resting state, removing free Ca+2 as its desorbed from its “cardinal site on troponin.”
“F-actin detaches from myosin heads,” and resynthesized ATP restores K+ preference.
K+ uptake and dissociation of cross-bridges between myosin heads and actin lead to muscle relaxation. F-actin “depolymerizes into the "profilamentous" form, polarizing water, and returns to the minimum-free-energy configuration of the resting muscle fiber.”
16.5 Agreements and Disagreements with Relevant Existing Knowledge
16.5.1. Electron Microscopic and X-Ray Diffraction Evidence
Electron micrographs have been used extensively to visualize relaxed muscle structures, particularly thin filaments. It is difficult to prove that observed thin filaments are a result of muscle activation during specimen preparation. Ling argues that electron microscopy techniques may naturally favor procedures enhancing image sharpness, potentially influencing the interpretation of the results. He introduces X-ray diffraction data to support his proposed model involving the polymerization of "profilamentous" actin into regularly arrayed F-actin filaments during contraction. X-ray findings show a hexagonal array of filaments present during contraction.
16.5.2. A Key Role of Cell Water in Muscle Contraction
Water movement, rather than thin filament movement, is the primary driving force in muscle contraction. Extracellular Na+ is critical for cell excitability. Hower, too much impedes cell contraction. “[M]uscle becomes unable to contract when it loses a part of its cell water after exposure to hypertonic solution (= excess solute, like Na+)” - “the loss of muscle contractility is not the consequence of loss of electrical excitability. Indeed perfectly normal action potentials persist in muslce no longer contracting in a hypertonic solution.” Hypertonic solutions enhance caffeine-induced contractions, “proving that the Ca+2 release mechanism remains intact.” [H]ypertonic solution[s] reduces the amount as well as the thermodynamic activity of water in the cell… one may deduce that… drastic reduction of water activity may… cause premature actin polymerization… [this] would be one possible reason for the widely observed stiffness of muscle treated with hypertonic solutions.”
16.5.3. A Mechanism That Prevents the Filaments from Tangling Up
Questions remain as to how filaments do not become tangled, especially during contraction, in the sliding filament model. In Ling’s model, thin filaments are kept in order at rest due to their “profilamentous” matrix. When actin becomes F-actin the cross-bridges hold it in place. Thick filaments, muscle fibers, and other cell structures “maintain their regular spacings” and are kept in place analogous to how cell volume is maintained - polarized multilayer water.
16.5.4. A Key Role of K+ Adsorption and Desorption in Muscle Contraction
In Ling’s model ATP adsorbs on cardinal sites on the myosin head which causes K+ adsorption on the myosin heads. “When ATP desorbs, K+ is liberated [during muscle contraction], as its adsorption sites now combine with actin sites, forming cross-bridges.” During each contraction K+ is released from the muscle into the blood. Intracellular Na+ does not appreciably change due to the intracellular salt bridges.
16.5.5. The Source of Energy and Force in Muscle Contraction
Albert Szent-Gyorgyi, Morales, Botts, and Ling all “regarded the function of ATP to be discharged through its binding onto the contractile protein, to maintain a relaxed state. Removal of ATP brings about contraction (= shortening).” ATP “keeps actin and the myosin heads from binding to each other” (= plasticizing). In the relaxed state, the cardinal adsorbent ATP does this by causing a conformation change that is the propagated along/ through myosin to keep beta- and gamma-carboxyl groups in their “K+-adsorbing state.” Contraction is when “desorption or hydrolysis of ATP leads to an autocooperative shift to the contracted state in which K+ is liberated and the beta- and gamma-carboxyl groups now form salt linkages with fixed cationic charges, including some on actin (or troponin-tropomyosin), and thus myosin and actin are bound together.”
This means “that ATP must exercise a stronger polarization effect on the contractile protein than does its hydrolytic product, ADP.” Research has shown the binding constant of ADP is “nearly a million times weaker” than ATP, giving credence to this idea.
There is missing energy in muscle contraction - ATP hydrolysis, CrP breakdown, glycolytic, and oxidative activities do not account for all observed heat production. Ling suggests the missing energy is ““stored”in the entire assembly and not in a particular compound or a particular chemical bond.”
Ling also provides an answer as to how the potential energy stored in the entire assembly is converted to perform mechanical work. He claims the osmotic force created due to the liberated K+, Na+, and Cl- and change in water polarization is sufficient for the tension.