Chapter 15 Oxidative Phosphorylation, ATP Synthesis, and Other Aspects of Mitochondrial Physiology
Rethinking the ETC: Embracing a Chemical Inductive Effect Over the Chemiosmotic Theory
As always, this is not medical advice, and reading this does not form a client relationship with me - your health is your responsibility.
Many have asked for my “thoughts” on things. The best place to start would be some of my older Substacks - especially the ones on supplementation (1 and 2). Also, please check out my IG story highlights. I have postponed consults for now; if you want to be added to my waitlist, please DM me on IG!
Today’s Substack will continue with Chapter 15, Oxidative Phosphorylation, ATP Synthesis, and Other Aspects of Mitochondrial Physiology. Chapter 14 discussed electrical potentials.
Summary
This is the current accepted view of oxidative phosphorylation. The respiratory chain, also known as the electron transport chain (ETC), is a series of protein complexes and electron carrier molecules within the inner mitochondrial membrane. These complexes play a crucial role in the process of oxidative phosphorylation, which is the final stage of cellular respiration. Cellular respiration is the process by which cells generate energy (ATP) through the breakdown of glucose.
In eukaryotic cells, including human cells, the respiratory chain is located in the inner mitochondrial membrane. The complexes of the respiratory chain include:
Complex I (NADH dehydrogenase): This complex receives electrons from NADH (produced during glycolysis and the citric acid cycle) and transfers them to the next complex.
Complex II (Succinate dehydrogenase): This complex is directly involved in the citric acid cycle and transfers electrons to the next complex.
Complex III (Cytochrome bc1 complex): It transfers electrons to cytochrome c.
Cytochrome c: A mobile carrier that transfers electrons between Complex III and Complex IV.
Complex IV (Cytochrome c oxidase): This complex is the final electron acceptor, transferring electrons to oxygen (O2) to form water.
There can be multiple complexes per ETC and multiple ETCs per mitochondria. The electrons move through these complexes in a series of redox reactions, and the energy released is used to pump protons (H+) across the inner mitochondrial membrane, creating a proton gradient. This proton gradient is then used by ATP synthase (Complex V) to produce ATP from ADP and inorganic phosphate (Pi), a process known as chemiosmosis.
The respiratory chain is often considered the "center" of cellular respiration because it is the site where the majority of ATP is generated in aerobic cells.
Mitochondrial oxidative phosphorylation of glucose leads to the greatest ATP yield - some will incorrectly state fatty acids lead to more ATP because they are not standardizing per carbon. ATP synthesis is coupled to oxidation by the mitochondria. There are multiple theories as to how this coupling occurs. ATP synthesis is accomplished in two steps via a “chemical inductive effect” - see below. “ATP functions in mitochondria as it does in cells generally, and maintains water in a state of polarized multilayers that tends to exclude solutes; in accord with the theory of volume regulation outlined in Chapter 13, ATP is noted to cause shrinkage and sucrose exclusion of mitochondria that initially lack ATP. Like ATP, uncouplers, ionophores, Mg2+, Ca2+, and ADP function as cardinal adsorbents, and may be classified according to whether their inductive effect is primarily electron-withdrawing or electron-donating.” The next chapter will explore muscle contraction and relaxation!
A mini-rant before I start: many continue to demonize cortisol. Cortisol, in and of itself, is not the issue. This especially holds for my understanding as I see hormones, neurotransmitters, etc., more as “residues,” but that is beside the point. Without cortisol, we would die in the case of hypoglycemia; it protects us from an over-immune, allergic, etc. response to things (all those with rashes, MCAS, autoimmunity, etc.), and the list goes on. It is clear those who continue to lay everything at its feet have never experienced or worked with someone who has adrenal insufficiency. Another consideration has to do with signaling - very much like euthyroid hypothyroidism, where one has “perfect by functional standards” labs but still experience the symptoms. In the case of thyroid, we know sufficient cortisol is needed for thyroid receptor action - another, are you hypothyroid, or is the synergistic cortisol being used to keep you alive? Cortisol-specific signaling without signs of primary, secondary, or tertiary adrenal insufficiency arguably has much to do with this chapter.
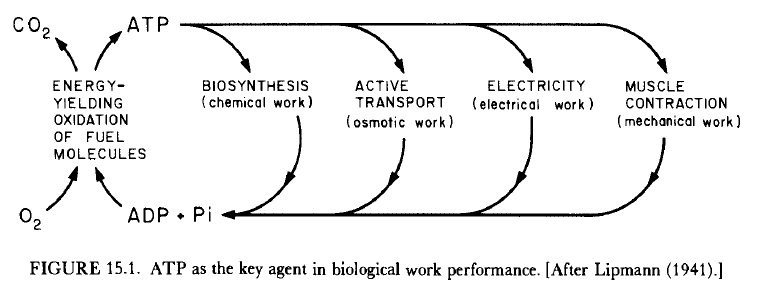
15.1 The Central Role of ATP in Biological Work Performance
Living cells require an uninterrupted energy source. Adenosine triphosphate, ATP, was designated as the cell’s energy carrier via its three phosphate bonds - acting as a potential energy biomolecule. Subsequent experiments questioned how “high-energy” ATP’s phosphate bonds are. Ion gradients and other alternate sources have been proposed as the living cell’s potential energy source.
15.2 The Sources of ATP
As shown in Figure 15.1, most processes involved in life depend on the hydrolysis of ATP. As a reminder, hydrolysis is when water breaks chemical bonds - and people continue to say the body water has nothing to do with these processes. Therefore, most biological processes act to “manufactur[e] proteins and other structural molecules,… and the conversion of food materials… into ATP.”
Immediate ATP sources include creatine phosphate (CrP) and arginine phosphate (ArP). Abundant in cells requiring rapid energy responses, these phosphagens play a crucial role. Creatine kinase catalyzes the Lohmann reaction, maintaining a steady ATP level as long as phosphocreatine is available.
“Under anaerobic conditions, glucose may be degraded into lactic acid (glycolysis) or ethanol (alcoholic fermentation). In these processes, two molecules of ATP are synthesized from each glucose molecule degraded. A much larger amount, however, is released by the total oxidation of glucose through respiration.”
Respiration, the primary ATP source, involves the tricarboxylic acid cycle and the electron transport-oxidative phosphorylation system within mitochondria. The cycle processes acetyl groups, ultimately yielding CO2 and hydrogen atoms. Electron-transferring reactions, catalyzed by dehydrogenases, lead to the reduction of oxygen, generating ATP at various points along the respiratory chain. The rate of ATP production is intricately tied to the level of ADP, showcasing respiratory control in mitochondria. The dynamic states of respiration highlight the responsiveness of ATP production to cellular needs.
“Electrons from the NADH-NAD+ system are at a high (negative) oxidation-reduction potential (Eh). Before its final reaction with oxygen, the electron pair gives off energy as it flows down the respiratory chain to yield three ATP molecules, one ATP at each of three different sites. Electrons from succinate, on the other hand, yield only two ATP molecules since they enter the chain at site II, further downstream.” - the higher FADH2:NADH from fatty acid oxidation causing reverse electron flow from NADH at complex I from glucose metabolism.
15.3 Theories of the Mechanism of Oxidative Phosphorylation and Their Critiques
Oxidative phosphorylation occurs in the mitochondria and is the primary producer of ATP in aerobic organisms. These organelles in various shapes play a significant role in metabolically active cells. Many do not realize that there is not only one mitochondrion per cell - “heart muscle, mitochondria may make up 50% of the cell volume; in rat liver they account for about 20%.” The inner membrane of mitochondria, impermeable to solutes, houses the respiratory chain components and exhibits infolding double-membrane structures called cristae. Another thing many do not realize is you can have multiple of the same complex type per each “electron transport chain.” The matrix, containing enzymes of the tricarboxylic acid cycle, fills the inner membrane and is gelatin-like in structure - because “~50% of its weight is proteins.”
There are multiple theories of the mechanism of oxidative phosphorylation:
The Chemical Coupling Hypothesis: suggests a common high-energy intermediate linking energy-yielding electron transfer reactions and energy-demanding ATP synthesis. However, the absence of a discovered intermediate and the unexplained requirement for an intact mitochondrial inner membrane challenges this hypothesis.
The Conformation Coupling Hypothesis: oxidative phosphorylation relates to ATP production through conformational changes in enzymes - strain → relaxed. Morphological changes observed in energized mitochondria supported this idea, but critics questioned the specificity of these changes.
The Chemiosmotic Hypothesis: this is the current theory governing the electron transport chain. An ionic gradient across the inner mitochondrial membrane provides the immediate energy source for ATP synthesis. The H+ gradient and a protomotive force derived from a pH gradient and an electrical potential across the inner membrane supposedly facilitate ATP production. This hypothesis gained attention and a Nobel Prize but faces substantial evidence against it - e.g., measured pH gradients are minimal, and the electrical potential is considerably smaller than predicted. It also relies on proton and electron tunneling - all those who love to espouse deuterium vs. hydrogen…
15.4 A Tentative Model of the Inductive-Associative Coupling Mechanism for Electron Transport and Oxidative Phosphorylation
ATP formation in two steps using inorganic phosphate, Pi, (=free phosphate anions in solution, solution = water mixture) and adenosine diphosphate (ADP):
Postulate one:
The ATPase (adenosine triphosphatase) is phosphorylated by Pi when Mg+2 and/or K+ are adsorbed onto the ATPase → ATPase is now a phosphoenzyme, EP.
The phosphoenzyme (an enzyme that has been phosphorylated = attach a phosphate group to a molecule or ion), EP, formed in the first step, phosphorylates ADP to produce ATP when Ca+2 and/or Na+ are adsorbed onto the EP.
Postulate two: The transition from adsorbing one set of ions to another is driven by a cooperative change in the adsorption characteristics, not by changing the concentration ratios of the ions but by altering the intrinsic equilibrium constant for the exchange of ions.
Postulate three: The components of the respiratory chain, which are structurally connected, also function together through a shared mechanism. This mechanism involves an indirect inductive effect, meaning that the functioning of one component influences the others. The idea is that there's a cooperative interaction among these components, ensuring they function together effectively.
Postulate four: There's a connection between the electronic state of the respiratory chain center and the behavior of the ATPase. The respiratory chain center, which can be either reduced (electron-rich) or oxidized (electron-poor), influences the ATPase. When the respiratory chain center is in a reduced state, specific ions like Mg2+ and/or K+ are more likely to be adsorbed by the ATPase. Conversely, when the respiratory chain center is in an oxidized state, different ions like Ca2+ and/or Na+ are favored for adsorption.
In simpler terms, the electronic state of the respiratory chain influences how the ATPase behaves in terms of ion adsorption. This, in turn, affects the production of ATP in a cyclic manner, as each oxidation and reduction cycle of the respiratory chain center leads to a change in the ion adsorption preferences of the ATPase, triggering the generation of ATP.
To summarize the four postulates, the connection between electron transport and phosphorylation involves cooperative shifts influenced by the inductive effect within the ATPase system. This allows for the step-by-step creation of EP (phosphorylated ATPase) and ATP from ADP + EP. The involvement of selective ionic adsorption is crucial in this process. The thesis is built on three key points:
The widespread occurrence of the inductive effect in chemistry.
The presence of cellular cations primarily in an adsorbed state.
The control of selective cation adsorption on proteins in living cells by cardinal adsorbents.
While this model shares little similarity with lipid-membrane pump-based chemiosmotic coupling mechanisms, it resembles other hypotheses in cell physiology, such as Slater's chemical intermediate hypothesis and the conformation hypotheses of Boyer and Green.
Model systems that indicate “whether oxidation and reduction of prosthetic groups can influence selective ionic adsorption”:
Heme-heme interaction and the Bohr Effect: Contrary to a purely mechanical interpretation, evidence suggests that regulating heme oxygen affinity by globin structure could involve inductive effects. In solution, hemoglobin exhibits flexibility inconsistent with a rigid mechanical model, challenging previous interpretations based on hemoglobin crystals.
Autocooperative Ion Adsorption Shifts Controlled by Oxidation-Reduction: “Oxygenation and deoxygenation, like oxidation and reduction, can indeed control the transition between the two cooperative states, and in each state many if not all of the functional groups have different properties.” “Ferrocytochrome binds only cations, including Mg+2, while ferricytochrome binds only anions, including Cl-, Pi, and ADP. Clearly, oxidation-reduction changes can alter ion binding on these proteins in an all-or-none manner.”
15.5 New Interpretations of Observations in Mitochondrial Physiology
Swelling and Shrinking
Role of ATP Concentration: Isolated mitochondria maintain their shape in a sucrose solution. Swelling occurs when ATP content falls below a critical level - again, “leaky gut and blood-brain barrier” considerations. The shrinkage of fetal liver mitochondria and enhanced respiratory control by ATP depend on ATP's presence and interaction with the mitochondrial inner membrane, not ATP hydrolysis - it is not about the “high-energy phosphate bond.”
Role of Mg+2 and Ca+2: Mg+2 and ATP may synergistically control water polarization. If Ca+2 displaces Mg+2 competitively, it reverses the effect of Mg+2 plus ATP on water structure. Swelling caused by hypotonic solutions (= low solute solution) doesn't uncouple oxidative respiration. Uncoupling oxidative phosphorylation is understood as the energy dissipating as heat vs. being used for ATP and water production. Ca+2 increases available space for solutes, and this effect is reversed by ATP and Mg+2, suggesting water depolarization by Ca+2 leads to swelling and ATP and Mg+2 induce long-range water polarization, causing shrinkage - some significant considerations when it comes to Ca-oscillations for exocytosis of neurotransmitters, hormones, etc. also, nerve-muscle action, why some do better with Mg and others Ca, etc.
Passive Osmotic Swelling: Isolated heart mitochondria swell in the presence of permeant anions and cations and absent ATP, indicating the opening of salt linkages between macromolecules inducing the swelling.
“Transport” of ATP: In the context of the AI hypothesis, the entry of solutes into the cell involves two modes: one through water polarized by proteins and the other via fixed ionic and other binding sites, providing greater specificity in permeability to ions and other solutes. Maintaining the polarized water surface barrier and specific ATP binding sites requires certain cardinal sites to be occupied by ATP. ATP and water are squeezed out of the mitochondria matrix where they are made when the matrix becomes condensed. Mitochondria readily take up ATP and sucrose when the water is “relatively depolarize[d].”
Thyroxine, T4, causes mitochondria swelling that is fixed with ATP and K+ - a clue as to why many do not do well with T4 and why giving thyroid should not be the first step at resolving issues.
The Effect of Ionophores, Uncouplers, and Other Cardinal Adsorbents on Mitochondrial Ion Distribution: Biologically active agents work similarly to Paul Ehrlich's lock and key model - the keys either open or close a door. Cardinal adsorbents primarily only do two things: donate or withdraw electrons. Thus, they are classified as electron-donating (EDC) or electron-withdrawing (EWC), affecting electron and positive charge density in mitochondria proteins. A stronger EDC can displace a weaker creating an EW effect as if an EWC were present, and vice versa for EWCs. Also, stronger EDCs can interact with anionic sites, EWCs with cationic sites, etc. Lastly, two cardinal sites can produce complex equilibrium effects - see my Substack on supplement equilibrium.
Uncouplers: “Uncouplers, [like DNP], are agents that "uncouple" oxidation from phosphorylation and at the same time activate ATPase activity and stimulate oxygen consumption.” Uncouplers seem to increase oxygen consumption, create heat, and activate ATPase activtiy by causing a loss of K+ and a gain of H+. In the case of DNP, respiration is stimulated at low concentrations and suppressed at higher. The more oxygen consumed, the greater the need for K+ - this again brings up the issue when many crave Na+ vs. K+ and need more oxygen to the cells via the Bohr/ Haldane effects, so the need for more CO2 via glucose metabolism.
In general:
“K+ depletion causes inhibition of respiration”
Increased K+, increases respiration
“Increase in the respiratory control index by ADP (i.e., increased O2 consumption) depends on K+, and loss of respiratory control parallels loss of K+, after which respiration stops.” - KCl restores.
Normal mitochondria balance K+ adsorption and desorption via Mg+2 and ATP which along with NAD+ “restore mitochondrial respiration and oxidative phosphorylation.”
A sufficient level of ATP is needed for respiration to continue.
The switch from the ATPase using Mg+2 and/ or K+ to Ca+2 and/or Na+ is required for oxidative phosphorylation to continue.
Thiols:
Ca+2 is lost, and Mg+2 is retained when succinate is oxidized - again, why we do not want to rely on the BCAAs. Add, biotin, and adenosylcobalamin are needed for this path.
Respiration favors Ca+2 accumulation over Mg+2.
Uptake of Ca+2 parallels NADH and NADPH cocnetration, a reduced state.
Inhibitors of respiration and uncouplers cause rapid Ca+2 release.
Thiol oxidation and respiration both remove electrons.
Selenite enhances “the deleterious effects of Ca+2 on mitochondria;” Mg+2 is protective.
DNP “favor H+ uptake at the expense of K+” and “ inhibit Ca+2 uptake.”
ATP: = electron-withdrawing cardinal adsorbent, EWC
ATP stimulates Ca+2 uptake into the mitochondria.
Supports “K+ accumulation in muscle.”
“Externally added ATP and respiration often have similar effects.” This is independent of ATP hydrolysis - one reason many do well with ATP supplementation, especially given its ability to rapidly enter the mitochondria matrix until a saturation point is reached; then, the matrix condenses sufficiently, and ATP and water are “squeezed out.”
ATP allows Ca+2 to displace H+.
ATP promotes water polarization.
ADP: = electron-donating cardinal adsorbent, EDC
Opposite effects of ATP.
Anaerobic mitochondria swelling reversed with ADP or DNP (= EDC).
ADP accelerates K+ loss.
ADP and anaeribic conditions inhibit Mg+2 release.
“Not only is the protein-water-ion system undergoing coordinated changes, but these changes are also synchronized in the time dimension.” - this makes the differential equation governing the processes a partial differential equation. We currently cannot solve PDEs exactly but instead use simplifications like boundary conditions, etc., and numerical methods. Physics often models a cow, humans, etc,. as cylinders because the boundary conditions “work.” And yet, people continue to cling to physics as if it has answered all these questions. At the end of the day, we are all oscillators.
The accumulation of hydroxyapatite in mitochondria that are “maximally loaded with Ca+2, i.e., in the presence of ATP.”:
Ca+2 is selectively adsorbed on anionic sites, displacing H+. Pi is adsorbed selectively as well.
Adsorbed Ca+2 and Pi → salt linkage dissociation.
Adsorbed Ca+2 activates ATPase → ATP hydrolysis → water depolarized → more Ca+2 and Pi enter mitochondria water.
ATP readsorbed → water polarized → Ca+2 and Pi precipitate as calcium phosphate.
The readsorbed ATP → Ca+2 and Pi readsorbtion → cyclical.
Ling theorizes the above process is how Ca+2 precipitates as oxalate.


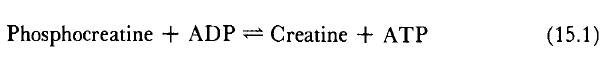


Interesting read!