Chapter 12: Permeability
Water, Ions, Amino Acids, and Sugars Dance Through Molecular Pores and Surface Secrets
As always, this is not medical advice, and reading this does not form a client relationship with me - your health is your responsibility.
Many have asked for my “thoughts” on things. The best place to start would be some of my older Substacks - especially the ones on supplementation (1 and 2). Also, please check out my IG story highlights. I am taking a few more consults, if you are interested, please message me on IG.
Today’s Substack will continue with Chapter 12, Permeability. Chapter 11 discussed Ling’s proposed mechanism as to how the cardinal adsorbents (e.g., Ca, ATP, insulin, etc.) lead to solute distribution. I highly recommend going over the figures in chapter 11 until you thoroughly understand them before moving on - they are the foundation of the association induction hypothesis in many ways.
Summary:
Living cells and tissues are readily permeable to water. “It is now clear that the permeability barrier to the movement of water is water itself, i.e., water polarized in multilayers within rather large “pores.” These pores are formed by amino acids, protein, and structured water at the cell surface. “The treatment of permeability of cells to ions, sugars, and amino acids by the association-induction hypothesis is based on three major concepts. First, the surface, or surface membrane, is in general a two-dimensional replica of the fixed-charge system that is the entire cell; as such, it contains interstices filled with water polarized in multilayers, and it contains proteins with potential adsorption sites for ions, sugars, amino acids, and other substances. Second, the passage of a solute into and out of the cell may in some cases be limited by its adsorption onto and desorption from macromolecules at the cell surface, and in other cases may be limited simply by diffusion through the water that is polarized in multilayers within the interstices.” Finally, the saturable (specific binding/ adsorption onto amino acids) and nonsaturable fractions (non-specific adsorption) depend on other ions which facilitate influx or efflux. “Insulin facilitates glucose entry into muscle inductively by creating adsorption site, and Na+ facilities amino acid uptake by the inductive modulation of adsorption sites at or near the cell surface.” Induction here means something is influencing another without direct physical contact.
12.1 Evidence Against the Conventional Lipoidal Membrane Theory
The inner mitochondrial membrane has a very high electrical conductivity, which contradicted the belief that it had a low ionic permeability. This is because things with high electrical conductivity should allow electric charges to move through them with minimal resistance.
A new belief emerged claiming K+-ionophores led to increased K+ permeability with no effect on Na+ permeability - the ionophores acting as a biological shuttle. There are either channel ionophores or carrier ionophores. Channel ionophores are said to create channels within the cell membrane to allow ion transport in and out. Carrier ionophores are said to be transport proteins that reversibly bind ions to transport them in and out of the cell. Comparing K+-specific ionophores on living cells to phospholipid bilayer compounds, in a K+-rich solution, “indicates that a relatively small percentage of the muscle cell surface is covered with a phospholipid bilayer.” This was corroborated using red blood cells too. This is important as red blood cells have the membranes with the highest lipid content. The search for natural K+ ionophores acting as shuttles was unsuccessful.
Membrane composition studies contained in Jain’s monograph showed that “in contrast to the variable and often very low lipid content, it is the proteins that constitute the more constant and abundant component. The missing water content also gives the false impression that lipids and proteins are the only compounds of the isolated membranes.” Mammalian erythrocytes, red blood cells, have just enough lipid content to form a continuous lipid bilayer all other cells do not. This is not accounting for the need to use lipids for other reasons within the cell - e.g., mitochondria inner membranes, etc. “The outer membrane of a mitochondrion is highly permeable and is widely regarded as not serving as a permeability barrier. The inner membrane, on the other hand, is regarded as far less permeable. Yet lipid analysis showed that it is the outer membrane that is rich in lipids (50% of dry weight), while the inner membrane is poor in lipids (20% of dry weight)… There is no continuous, uninterrupted lipid bilayer forming backbone structure in these membranes and that lipids occur only as islands or patches in the membrane.”
Electron microscope imaging of defatted showed the opposite of what was expected compared to control imaging. “Since after the removal of the lipids the thickness of the whole membrane does not change, one can only conclude that lipids exist in pockets or islands and that it is the total area of the membrane that undergoes shrinkage… There is evidence that it may be simply the proteins, which as shown in [Jain’s monograph], are the most constant component of all cell membranes and hence a more reasonable candidate for the widely observed trilaminar structure” seen by the electron microscope. The trilaminar structure is another term for lipid bilayer. Electron micrographs of only protein materials show the same trilaminar structure, further supporting this hypothesis.
Also, if any part of the cell were to ripped off, you should see the contents spewing out until the individual pieces' membrane is restored- assuming there is enough lipid to support the surface area change in both segments. However, this is not seen.
12.2 What is the Rate-Limiting Step for the Entry of Water into Living Cells?
Contents of a living cell, or protoplasm, can be isolated and suspended in an aqueous medium without dissolving and can be excited to create action potentials. This is occurs without the cell membrane regenerating. This is like how water droplets act when they come in contact with another droplet and with bulk water. “So molecules at the surface of the protoplasm may also redistribute and reorganize themselves in such a way as to yield qualities that are usually considered surface or membrane properties. But the cell surface in essenece is nonetheless of the same chemical composition as the bulk phase protoplasm: It is primarily a protein-ion-water system containing varying amounts of phospholipids… It is conceivable that in some casues the rate of diffucsion of molecules through the cell surface and within the cell interior may take place at similar rates.” Experiments confirm that diffusion through the cell interior are similar in magnitude as those through the cell surface - both are slower than diffusion through normal water. This also means the cell surface is primarily water.
12.3 Polarized Water as the Semipermeable, Selective Permeability Barrier
“According to the AI hypothesis, it is water polarized in multilayers by cell-surface proteins that serves as a continuous semipermeable barrier, at once separating the living cell from its environment and maintaining a continuity essential for its functional activities.” The “total area of polarized water at the cell surface and the degree of polarization of this water,” will be important factors moving forward as these will dictate cellular action.
12.4 Permeability of Cells to Ions
I have spoken about how divalent cations inhibit uptake, etc. of other divalent cations (same idea for the monovalent too). However, Ling points out an important fact that “K+ does not inhibit Rb+ entry, as one would expect… Instead, K+ facilitates Rb+ entry. A similar facilitory effect of Na+ on Rb+ entry [is] also observed.” This is an important distinction because many will point to competition vs. the actual observed facilitation - in many ways it’s the difference between a finite vs. infinite game worldview. This also has important implications for heavy metal, etc. “detoxification.” This effect is called the “triplet mechanism.” When the adsorption energy on the surface adsorption sites is high, a second free cation can assist with the desorption from the surface site to intracellular sites. “If the second cation comes fro the same side of the cell surface as the entering ion, it is referred to as the biliard-type triplet adsorption-desorption route. If the second free ion comes from the phase that the first is entering, it involves a liberation motion and is called the pinwheel-type triplet adsorption-desorption route.”
As a reminder, “the major fixed anions on proteins are the beta- and gamma-carboxyl groups belonging, respectively, to aspartic and glutamic acid residues.” These sites are where K+, Na+, etc. are adsorbed. “The most reasonable candidates for the fixed cationic sites would be those provided by the epsilon-amino groups of lysine residues and the guanidyl groups of arginine residues of cell surface proteins” - these sites are where anions (negatively charged ions like Cl-) would adsorb. The balance of lysine and arginine bring up interesting follow-on questions having to do with “viruses” and “viral-reactivation.” P5P, the “active” form of vitamin B6 blocks anion adsorbtion in red blood cells - a further clue to “B6-toxicity” and high serum B6 levels.
Radioisotopes are also used to study ion efflux.
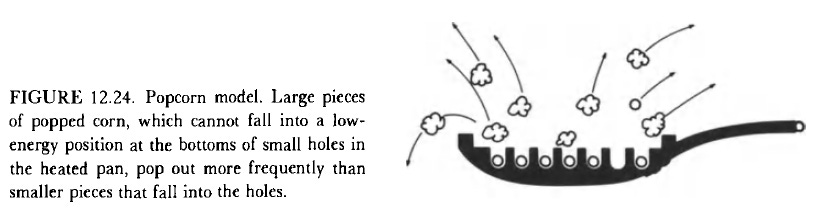
As a reminder, “the bulk of cell K+ is preferentially adsorbed. Half of cell Na+ is also adsorbed but less tightly than K+; the remainder is free and dissolved in cell water. Therefore cell Na+, free and adsorbed, exists in a higher-energy state than cell K+. The popcorn analogy… illustrates this point; small pieces of popped corn (like K+) find themselves at the bottom of the lower-energy wells and do not bounce out of the pan as fast as the larger ones (like Na+). This explains why K+ efflux is much slower than Na+ efflux. On the other hand, if we regard an empty pan as a model of the cell surface and scoop up from a pan containing equal numbers of large and small pieces of popped corn, we would have collected more small pieces than large ones, because the spaces represented by the narrow pits (anionic adsorption sites) are available to small ones (K+) but not to large ones (Na+). This explains why K+ is more “permeable” than Na+ from influx measurements.” - Na+’s hydrated diameter is larger than K+. The slower K+ efflux is seen with the delayed potassium outward current during action potentials.
Certain can also facilitate other ion efflux like K+ facilitates Rb+ influx. The rate “depends on both the concentration and nature of the similar ions” around the fixed sites.
K+ and Na+ efflux in human lymphocytes show a biphasic response - a fast and slow fraction. The fast fraction would be from the free, nonsaturable fraction and the slow from the adsorbed, saturable fraction. For more information on the transient inward current and delayed outward current seen in action potentials (e.g., used by neurons, etc.), please see this bioelectricity video - the entire course is a good introduction to the mainstream view of action potentials, how neurons function, and how the Hodgkin-Huxley model was arrived at.
Ouabain, made from the constituents of strophanthus, a cardiac glycoside that increase the heart’s output force while decreasing its rate of contraction, or low extracellular K+, “change the system in such a way that cell-surface Na+ takes on the characteristics of surface K+ in normal cells. The surface anionic sites now prefer Na+ over K+, and Na+ entry and exit both become surface-limited.” Again, increased intracellular Na+ acts as a “band-aid” in a low K+ and ATP state.
12.5 Sugar Permeation and Its Control by Insulin
Insulin increases the amount of glucose that can be absorbed by muscle cells. However, insulin is not needed for glucose uptake into the muscle cells - this is where the notion of other glucose transporters came about. This is also why, glucose and insulin spikes are not the reason for obesity, etc.
12.6 Amino Acid Permeation and Its Dependence on External Na+
Insulin also increases the amount of amino acids that can be absorbed by muscle cells. There are leucine-preferring (L-mediation) or alanine-preferring (A-mediation) of neutral amino acid uptake. “Almost every neutral amino acid has affinity for both mediating systems; methionine, in particular, has strong affinities for both systems” - arguably because methionine is needed for S-adenosylmethionine, the universal methyl donor.
12.7 Surface Protein Adsorption Sites as the Seat of the Selective Adsorption-Desorption Route for Entry of Amino Acids
For amino acids to enter the cell, they must “first combine with the surface adsorption sites.” These sites are the ones that prefer Na+ to K+ - this is why Na+ is shown to come in with amino acids and K+ is excreted (the Na+, K+ oscillations I frequently discuss). If the concentration of extracellular Na+ is decreased or the extracellular K+ is increased (the K+ competes for these surface adsorption sites), less amino acids will adsorb. This is a big reason why people do well when they have salt with their protein; the same applies for glucose. The amino acids are then desorbed into the cell or back to the extracellular environment when K+ displaces the “surface-adsorbed Na+.” Therefore, intracellular amino acid “adsorption sites are favored by the protein existing in the K+ state” - where the surface adsorption sites are favored by the protein existing in the Na+ state. The surface and intracellular adsorption sites require ATP for proper folding/ unfolding.